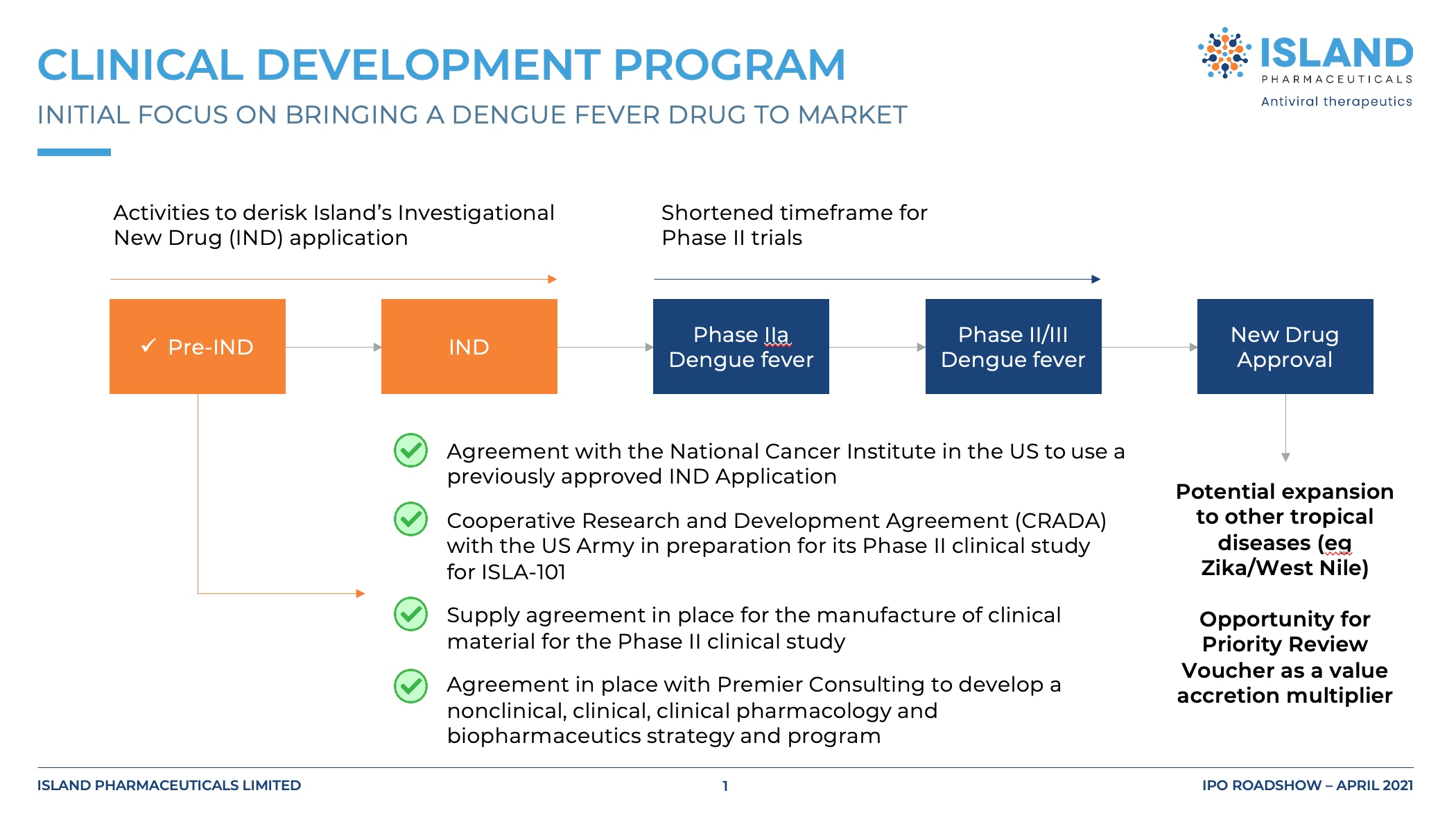
Clinical programme
Island's clinical programme has achieved significant progress. In August 2024, the US FDA cleared the company's proposed amendment to the ISLA-101 Phase 2 clinical trial protocol, structuring the challenge study as a Phase 2a/b trial with prophylactic and therapeutic arms across two cohorts.
In October 2024, Island announced dosing of the Phase 2a prophylactic (preventative) cohort. Weeks later, in November 2024, Island announced the initial data readout from the Phase 2a clinical trial. Results confirmed the safety profile of ISLA-101, and evidence of its anti-dengue virus activity (reduction of viral load in blood). The Safety Review Committee further noted that blood levels of ISLA-101 were seen as expected, and given positive safety and efficacy signals, recommended that Island proceed with the therapeutic Phase 2b cohort.
In January 2025, Island completed enrolment of all subjects in the Phase 2b therapeutic cohort. High-level results from the Phase 2b study are anticipated to be available around April 2025, with full results from the unblinded data of both the Phase 2a and Phase 2b cohorts expected in Q4 FY2025.
Infectious Disease expert Prof. Stephen Thomas MD, explains what is a Human Challenge Trial.
“When there are no available animal models of a disease, researchers take a weakened form of the virus and create a mild, safe form of the disease in a healthy person. The idea is that in a safe, well-controlled environment using a small number of people, we can get information that normally would involve going into the field and exposing a lot more people.
It is good timing for Island to test their product ISLA-101, now that it has successfully passed the Phase 1 safety study. The Phase 2 trial then can examine, in a small, very controlled and safe environment, if ISLA-101 has an impact on viral replication in humans, the symptoms, and the immune response.”
Professor Stephen Thomas MD
Director, Update Global Health Institute
Detailing how volunteers are set to be experimentally infected with a weakened form of the virus to analyse the impact and to assess the development of a drug or vaccine.
Human challenge trials are regarded by some as a faster way to develop new drugs and vaccines.
.png)
